US Quiz of the Month – Novembro 2023
Case Report
We present a case of a 75-year-old woman with jaundice, fatigue and weight loss for three weeks. Her blood tests showed hyperbilirubinemia with cholestasis and moderately elevated liver enzymes (total bilirubin 8.29 mg/dL, direct bilirubin 5.98 mg/dL, aspartate aminotransferase 180 IU/mL, alanine aminotransferase 171 IU/mL, alkaline phosphatasis 1540 IU/mL, gamma-glutamyl transferase 1360 IU/mL). The patient underwent an abdominal ultrasound, which revealed gallstones and marked common bile duct (CBD) and main pancreatic duct (MPD) ectasia (“double-duct” sign). Solid lesions were not identified in computed tomography scan.
She was referred for endoscopic ultrasound, which showed ectasia (11 mm) of the MPD with an echogenic 10 mm nodule adherent to the duct wall on its periampullary portion, without acoustic shadowing (Fig. 1). The CBD was markedly dilated (17 mm) (Fig. 2) until ending abruptly at the ampullary region. There were no enlarged lymph nodes or signs of vascular or organ invasion.
Duodenoscopy showed a protruded Vater’s papilla with an irregular wide orifice from which mucus emerged (“fish-eye” sign) (Fig. 3). Transpapillary biopsies were taken. Endoscopic retrograde cholangiopancreatography with sphincterotomy was done and biliary (10 Fr x 10 cm) and pancreatic (5 Fr x 4 cm) plastic stents were placed.
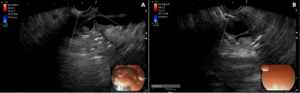
Figure 1.A,B- Main pancreatic duct and periampullary nodule.
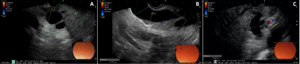
Figure 2.A,B- Common bile duct and main pancreatic duct ectasia at the periampullary region; C- Main pancreatic duct ectasia at the pancreatic neck.
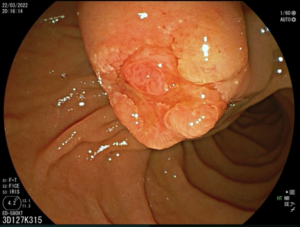
Figure 3. Protruded Vater’s papilla.
WHAT IS THE MOST LIKELY DIAGNOSIS?
Discussion:
Biopsies confirmed a poorly differentiated adenocarcinoma G3, with an immunohistochemistry profile suggestive of biliopancreatic origin [CK7+, CA19.9+, CDX2+ (weak), CK20-] (Fig. 4). The case was discussed in a Multidisciplinary Team Meeting and a cephalic duodenopancreatectomy was proposed.
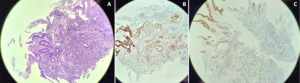
Figure 4.A- Poorly differentiated adenocarcinoma G3 on hematoxylin-eosin stain (50x magnification); B- Immunohistochemistry expressing CDX2 (weak positive) (100x magnification); C- Immunohistochemistry negative for CK20 (100x magnification).
Intraductal papillary mucinous neoplasms (IPMN) are intraductal epithelial neoplasms of mucin-producing cells which arise in the main pancreatic duct or its branches1. They present as a cystic lesion with associated dilatation of the main or branch pancreatic ducts and their cytomorphological analysis shows papillary fragments of mucinous epithelium in a background of abundant thick extracellular mucin, a hallmark feature2.
Main duct (MD) IPMNs result in an abrupt dilation of the main pancreatic duct and can be associated with a “fish-eye” papilla extruding mucin during endoscopic examination, which is a pathognomonic feature3. Additionally, they can also display the “double-duct” sign (combined dilatation of the MPD and CBD), an infrequently encountered finding on pancreatobiliary system imaging, most commonly associated with pancreatic head cancer or choledocholithiasis4. In jaundiced patients with “double-duct” sign, a malignant cause is likely, whereas in the absence of jaundice benign causes are more frequently seen4.
MD-IPMNs have a variable malignant potential ranging from 38–68%3. Not only a proportion of these evolve over time to become malignant, but also patients with IPMN are at an increased risk of developing conventional pancreatic ductal adenocarcinoma (PDAC) elsewhere in the pancreas, therefore surveillance even after resection is recommended 5.
PDAC derived from an IPMN is called intraductal papillary mucinous carcinoma (IPMC), which has distinct clinical and histopathological characteristics compared to conventional PDAC1,6. IPMC has a more indolent course and less aggressive histopathological features than PDAC, which can be related to its earlier detection among an IPMN surveillance program6. Its least common histological precursor, the pancreatobiliary type, is associated with a more aggressive course and invasive carcinoma5.
The management of IPMNs requires a multidisciplinary approach and the combination of many different diagnostic modalities to reach a diagnosis and provide adequate treatment when needed.
References
- Mas L, Lupinacci RM, Cros J, Bachet JB, Coulet F, Svrcek M. Intraductal papillary mucinous carcinoma versus conventional pancreatic ductal adenocarcinoma: A comprehensive review of clinical-pathological features, outcomes, and molecular insights. Int J Mol Sci. 2021;22(13):1–19.
- Geramizadeh B, Marzban M, Shojazadeh A, Kadivar A, Maleki Z. Intraductal papillary mucinous neoplasm of the pancreas: cytomorphology, imaging, molecular profile, and prognosis. Cytopathology. 2021;32(4):397–406.
- Rangwani S, Juakiem W, Krishna SG, El-Dika S. Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review. Diagnostics. 2023;13(4):1–14.
- Sinha R, Gardner T, Padala K, Greenaway JR, Joy D. Double-duct sign in the clinical context. Pancreas. 2015;44(6):967–70.
- Tanaka M, Fernández-del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Vol. 17, Pancreatology. International Association of Pancreatology and European Pancreatic Club; 2017. 738–753 p.
- Aronsson L, Bengtsson A, Torén W, Andersson R, Ansari D. Intraductal papillary mucinous carcinoma versus pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Int J Surg. 2019;71(9):91–9.
Authors
Maria Inês Canha1 Gonçalo Ramos 1,2 Sara Turpin 3 Paulo Jorge Ribeiro 2
- Gastroenterology Department, Centro Hospitalar Universitário de Lisboa Central, Lisbon, Portugal
- Gastroenterology Department, Hospital do SAMS, Lisbon, Portugal
- Pathology Department, Hospital do SAMS, Lisbon, Portugal


