US Quiz of the Month – November 2020
CASE REPORT
A 68-year-old female patient presented with nonspecific abdominal pain and early satiety. Laboratorial tests revealed normal liver function and carbohydrate antigen 19-9 (Ca19.9), as well as an elevated serum amylase (163 U/L). The patient had a prior history of hypertension, type 2 diabetes mellitus and dyslipidemia. An abdominal computed tomography (CT) scan showed a pseudonodular area measuring 12x11mm (axial), with arterial phase hyperenhancement, located in the anterior part of the transition between the body and tail of the pancreas (Fig. 1).
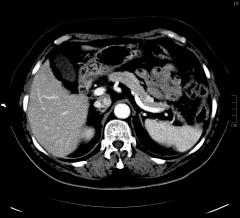
Figure 1. CT scan (axia showed a pseudonodular area of 12x11mm (axial), with arterial phase hyperenhancement, on the anterior part of the transition between the body and tail of the pancreas.
Endoscopic ultrasound (EUS) identified a hypoechoic, homogeneous, round, vascular, well-circumscribed solid nodular lesion, measuring 10×10 mm (Fig. 2 and 3).
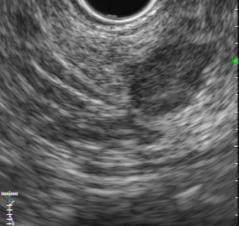
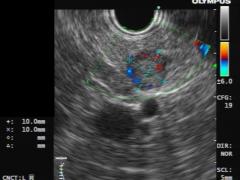
Figure 2 and 3 – EUS identified a hypoechoic, homogeneous, round, vascular, well-circumscribed solid nodular lesion, measuring 10×10 mm.
The lesion did not communicate with the main pancreatic duct. An EUS-guided fine-needle aspiration (FNA) using a 25G needle was performed with three passes (Fig. 4).

Figure 4 – EUS-guided fine-needle aspiration of the lesion was performed.
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
FNA cytology showed small clusters of cells with salt-and-pepper chromatin nuclei, positive for cytokeratin AE1/AE3 and synaptophysin. The findings were compatible with pancreatic neuroendocrine tumor (pNET). Immunocytochemistry revealed a Ki67 index <1%. The patient also had an elevated serum chromogranin A (108 ng/mL). Positron emission tomography (PET 68Ga-DOTANOC) confirmed a hypermetabolic nodular lesion, without further enhancements. Considering the size and grade of the tumor, the patient was proposed for surveillance. However, considering the patient individual decision, a distal pancreatectomy with complete resection of a non-functional grade 1 pNET was performed.
Increasing numbers of pNET are being diagnosed incidentally during radiologic or endoscopic studies [1]. EUS with FNA is an effective method to provide an accurate identification of pNET, although contrast-enhanced ultrasonography may improve the diagnosis capacity [2]. Moreover, immunocytochemical studies offer additional information, allow grading of the tumor and support differential diagnosis between other pancreatic neoplasms and metastatic lesions [3]. However, optimal management of asymptomatic, small (<2 cm) and low grade pNET still remains controversial [4].
REFERENCES
- Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Ann Surg Oncol. 2012;19(9):2932
- Falconi M, Bartsch DK, Eriksson B, et al. Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95(2):120-34.
- Salaria SN, Shi C. Pancreatic Neuroendocrine Tumors. Surgical Pathology Clinics. 2016;9(4):595-617.
- Falconi M, Eriksson B, Kaltsas G et al. Consensus guidelines update for the management of functional p-NETs (F-p-NETs) and non-functional p-NETs (NF-p-NETs). Neuroendocrinology. 2016; 103(2): 153–171.
AUTHORS
Margarida Flor de Lima1, Nuno Nunes1, Diogo Bernardo Moura1, Vera Costa Santos1, Maria Antónia Duarte1.
- Gastroenterology Department, Hospital do Divino Espírito Santo de Ponta Delgada, Ponta Delgada, Portugal.


