US Quiz of the Month – July 2019
CASE REPORT
A 50-year-old female patient without significant past medical history, presented with isolated GGT elevation (135U/L) and weigh loss (3Kg) in the past 6 months. Abdominal and pelvic MRI revealed a well-defined, round, hypervascular solid lesion, with 10mm, located in the pancreatic tail (Fig. 1). She was then referred to our clinical center.
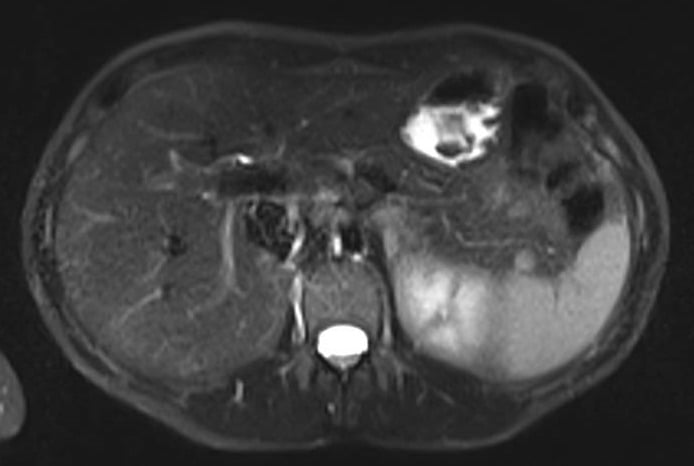
Figure 1. Abdominal and pelvic MRI – T2 (axial view): 10mm, well-defined, round, hypervascular solid lesion, located in the pancreatic tail.
A 99mTc-sulfur colloid scintigraphy was done and did not show any uptake in the location of the lesion previously described (Fig. 2).
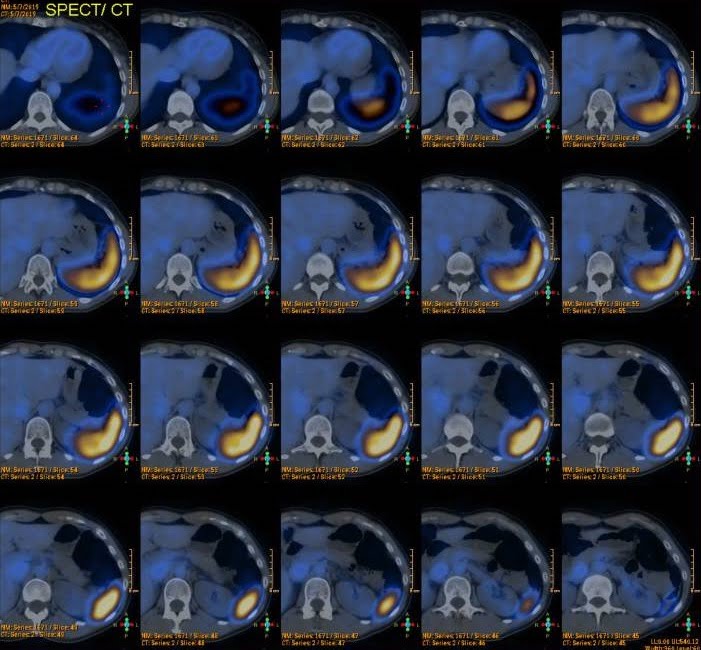
Figure 2. 99mTc-sulfur colloid scintigraphy: no uptake in the location of the pancreatic lesion previously described.
An EUS was therefore performed and a 12x7mm hypoechoic lesion was identified in the pancreatic tail. It was a round-shaped, well-defined, homogeneous intrapancreatic mass (Fig. 3). The lesion characterization was complemented by EUS-elastography, which displayed a homogeneous green elastographic pattern (Fig. 4). The remaining pancreas was unremarkable. EUS-FNB (Acquire – Boston Scientific; 25G, 1 pass) was performed.
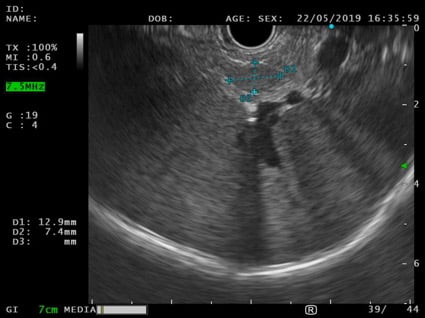
Figure 3. EUS (transgastric view): 12x7mm round-shaped, well-defined, hypoechoic, homogeneous lesion in the pancreatic tail.
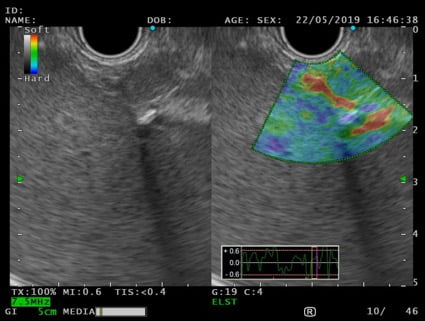
Figure 4. EUS – elastography (transgastric view): pancreatic solid lesion, with homogeneous green elastographic pattern.
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
Cytoblock showed groups of CKAE1-AE3 negative and CD45 positive cells, supporting the diagnosis of an intrapancreatic accessory spleen (Fig. 5).
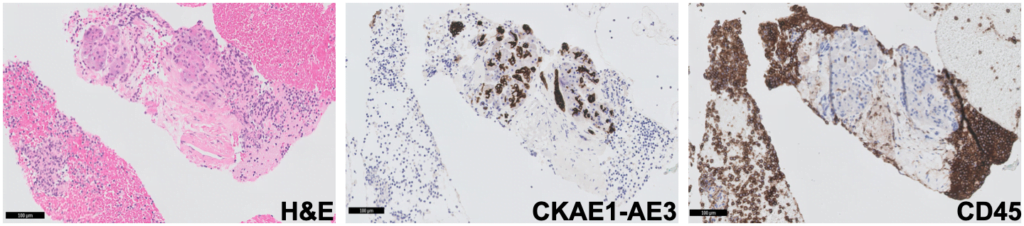
Figure 5. Pathology – cytoblock (H&E; CKAE1-AE3; CD45): groups of CKAE1-AE3 negative and CD45 positive cells.
Accessory spleen is a common benign congenital anomaly, with an approximate prevalence of 10–30% [1]. The most frequent location is the splenic hilum (80%), followed by the pancreatic tail (17%) [2]. Intrapancreatic accessory spleen (IPAS) are usually diagnosed incidentally on abdominal US, computed tomography, or magnetic resonance imaging. Its hypervascular nature and imaging characteristics similar to the adjacent spleen helps support the diagnosis [3]. However, as a well-defined homogeneous and hypervascular lesion, it is most frequently misdiagnosed as pancreatic NET. Consequently, many patients undergo an unnecessary pancreatic resection. 99mTc-sulfur colloid scintigraphy is a highly sensitive and specific noninvasive test used to confirm IPAS diagnosis. Nevertheless, false negative may occur, as in this case report, especially for lesions less than 10mm.
In this situation, EUS can be very useful to achieve a diagnosis. Endosonographically, IPAS is a round-to-oval-shaped, small lesion, usually less than 2cm, with well-defined margins, a homogeneous echotexture, and an echogenicity lower than the adjacent pancreas and identical to that of the spleen. EUS Doppler mode may confirm its increased vascularity [1, 4]. As a hypervascular pancreatic lesion, using contrast-enhanced EUS, IPAS typically shows a marked and homogeneous hyperenhancing appearance during the arterial phase compared with the surrounding pancreatic parenchyma [4, 5]. EUS elastography may be also useful to support IPAS diagnosis. Benign pancreatic masses, such as IPAS, have increased elasticity compared to malignant ones, therefore displaying a green elastographic pattern [6]. EUS-FNA can also be performed and is safe and effective, confirming the diagnosis in 90% of the cases and with no complications reported [7]. The cytologic features of the splenic tissue are characterized by small lymphocytes with a mixed inflammatory infiltrate with the appearance of the white pulp and presence of thin-walled blood vessels, which represent the splenic sinuses. When cell block or histology is available, confirmation can be made by CD8 immunohistochemical staining, a commonly used T-cell marker that is taken up specifically by endothelial cells of the splenic sinus [1]. Moreover, a negative staining with chromogranin and low-molecular-weight cytokeratin rules against NET.
In conclusion, when an asymptomatic pancreatic mass is detected, IPAS diagnosis should be considered, especially if the lesion has the same imaging features as the spleen. Because it is crucial to accurately differentiate IPAS from pancreatic neoplasms, EUS-FNA should be performed to ensure the correct diagnosis and management, with avoidance of unnecessary surgery.
REFERENCES
- Krishna SG, Heif MM, Sharma SG, Pandey T, Rego RF. Intrapancreatic accessory spleen: investigative dilemmas and role of EUS-guided FNA for diagnostic confirmation. JOP. 2011;12:603–606.
- Halpert B, Gyorkey F. Lesions observed in accessory spleens of 3,011 patients. Am J Clin Pathol. 1959;32:165–168.
- Okun SD, Lewin DN. Non-neoplastic pancreatic lesions that may mimic malignancy. Semin Diagn Pathol. 2015;33:31–42.
- De Robertis R, D’Onofio M, Manfrin E, et al. A rare case of pancreatic head splenosis diagnosed by contrast-enhanced ultrasound. Ultraschall Med. 2014;35:72–74.
- Will U, Arciadacono P, Petrone MC, et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933–940.
- Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Munoz JED. Quantitative endoscopic ultrasound elastography : an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172–1180.
- Ardengh C, Lopes V, Kemp R, Lima-Filho ER, Venco F. Pancreatic splenosis mimicking neuroendocrine tumors: endoscopic ultrasound guided fine needle aspiration. Arq Gastroenterol. 2013;50:10–14.
AUTHORS
Susana Marques1, Miguel Bispo1, Ricardo Rio-Tinto1, Paulo Fidalgo1, Mireia Castillo-Martin2, Jacques Devière 1,3
- Gastroenterology Department, Champalimaud Foundation, Lisbon, Portugal.
- Pathology Department, Champalimaud Foundation, Lisbon, Portugal.
- Gastroenterology Department, Erasme University Hospital, Brussels, Belgium.


