US Quiz of the Month – March 2019
CASE REPORT
A 65-year-old woman presented with new onset diabetes. Hes past medical history was remarkable for headache, anxiety and vasomotor symptoms for the last 3 years. Abdominal ultrasound followed by contrast-enhanced abdominal computed tomography (CT) revealed a 14-cm enhanced heterogeneous intra-abdominal mass, with cystic areas, in contact with the posterior wall of the gastric fundus (Figure 1).

Figure 1. Abdominopelvic CT (axial and coronal view): a 14-cm enhanced heterogeneous intra-abdominal mass, with cystic areas, in contact with the posterior wall of the gastric fundus.
Endoscopic ultrasound (EUS) was performed and showed a giant well-defined rounded hypoechogenic mass, without cleavage plane between the mass and the gastric wall. This was a very heterogeneous mass, with anechoic areas, suggestive of cystic transformation. Its size exceeded the endoscopic field (Figure 2-4).
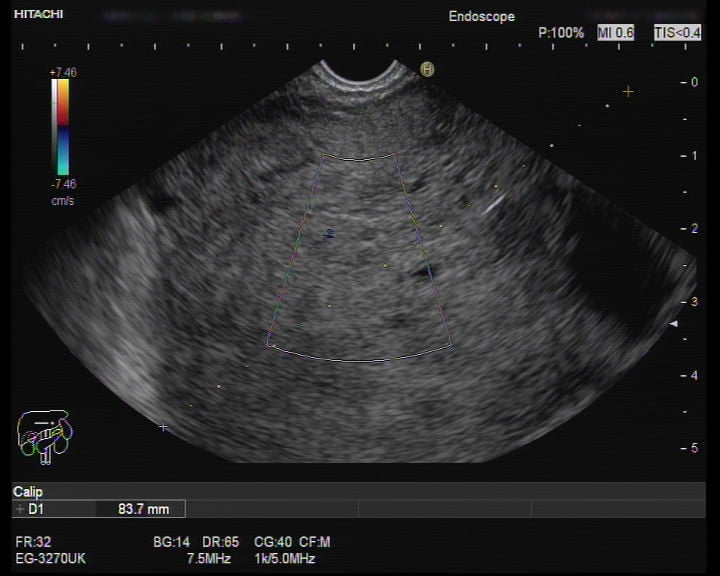
Figure 2. EUS (transgastric view): a giant well-defined rounded hypoechogenic and heterogeneous mass, with anechoic areas, suggestive of cystic transformation.
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
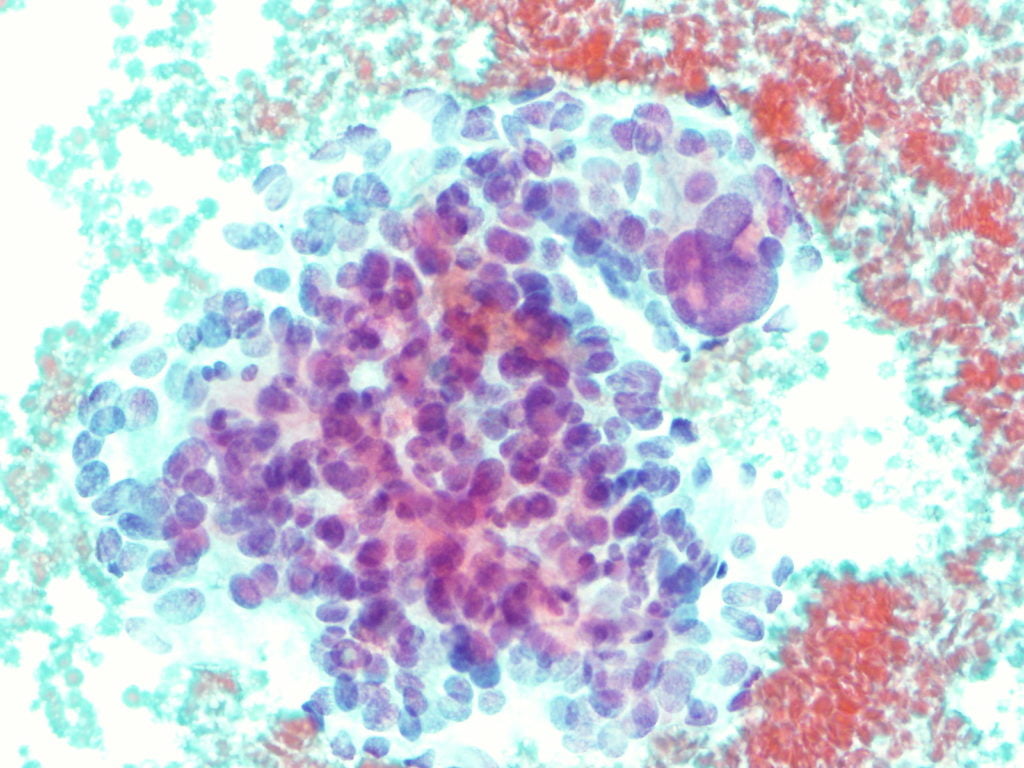
EUS-fine needle biopsy (EUS-FNB) using a 25G needle with rapid on site cytological examination (ROSE) was performed. Cytological analysis performed on the cell block demonstrated irregular clusters of cells with finely eosinophilic granular cytoplasm and pronounced anisokaryosis with large and irregular nuclei (Figure 5). Immunohistochemistry was positive for vimentin and synaptophysin and negative for CD117, S100 and CD34; Ki67<3%.
Toward these findings, 24-hour urine fractionated metanephrines were measured and were elevated (normetanephrine 5832pg/mL and metanephrine 11738pg/mL) and a 123I-meta-iodobenzylguanidine Single Photon Emission Computed Tomography (123I-MIBG SPECT/TC) was performed and revealed an intense radioisotope uptake from the retroperitoneal mass, with a central photopenic area suggestive of necrosis, indicative of a tumor originated from the embryonic neural crest tissue, without evidence of metastasis (Figure 6).
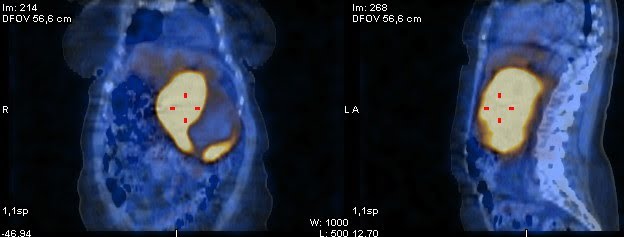
Figure 6. Radioisotope imaging revealed a bulky retroperitoneal mass with intense radioisotope uptake, suggestive of a tumor originated from the embryonic neural crest tissue.
Patient went surgical resection and histology revealed an encapsulated 16x16x10-cm tumor, weighing 1363mg, with a trabecular pattern of cells within a prominent vascular network, round and oval cells and giant multinucleated cells with abundant granular eosinophilic cytoplasm, without vascular invasion. Immunohistochemistry was positive for chromogranin and synaptophysin and focally positive for S100; Ki67<15%. The final diagnosis was secretory retroperitoneal paraganglioma.
Paragangliomas are rare neuroendocrine tumours arising from the neural crest tissue that develops into sympathetic and parasympathetic paraganglia throughout the body1. Extra-adrenal paragangliomas account for only 10 to 15% of all paragangliomas, with the intra-abdominal cavity being the most common site of extra-adrenal occurrence1. Functional paragangliomas have shown to secrete norepinephrine and nor-metanephrine resulting in clinical manifestations such as fluctuating or episodic hypertension, headache, and sweating1. On abdominal CT, there are no unique imaging characteristics specific for paragangliomas; consequently, these tumors may be mistaken for other primary epithelial or mesenchymal abdominal tumors. Advancement in the clinical application and use of EUS in recent years has transformed the field of gastroenterology, with the ability to identify and manage a wide variety of disorders, even extending beyond the gastrointestinal tract. Beyond ultrasound characterization, EUS allows guided biopsies, providing “core” specimens that preserve tissue architecture for histological diagnosis and immunostaining.
Here, we report a case of retroperitoneal mass successfully diagnosed as a paraganglioma using EUS-FNB without any complications. As illustrated by the present case, paraganglioma should be included in the differential diagnosis of a retroperitoneal mass at EUS, particularly if the mass is cystic, despite its rarity2. Nevertheless, careful should be taken when a needle puncture of a suspected functioning paraganglioma is performed, since catecholamine may be release triggering and adrenergic crisis2,3. Due to the malignant potential of paragangliomas, surgical excision is the preferred management1.
REFERENCES
- Brahmbhatt P, Patel P, Young M, et al. Retroperitoneal Paraganglioma Presenting as a Chest Pain: a case report. Case Reports in Oncological Medicine. 2013; 2013.
- Akdamar M, Eltoum I, Eloubeidi M. Retroperitoneal paraganglioma: EUS appearance and risk associated with EUS-guided FNA. Gastrointestinal Endoscopy. 2004; 60:1018-1021.
- Endoscopic Ultrasound-Guided Fine Needle Biopsy For The Diagnosis Of Paraganglioma. Program No. P541. World Congress of Gastroenterology at ACG2017 Meeting Abstracts. Orlando, FL: American College of Gastroenterology.
AUTHORS
Catarina Félix1, Susana Marques1, Martinha Chorão2, Cristina Chagas1
- Gastroenterology Department, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal.
- Pathology Department, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal.


